
| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |

| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |
We began by reminding everyone that the final exam is cumulative, and that an optimal study strategy would include the review of past exams (see Exam 1 and Exam 2). The answers seen are tabulated on the answer keys. Questions that almost everyone got correct are not likely to reappear on the final, but questions that a substantial portion of the class missed are excellent candidates for recycling on the final exam.
We began by examining a figure from the textbook showing different potential modes of regulation of gene expression, shown below. We might regulate gene expression by controlling transcription, controlling translation, or controlling protein function post-translationally.

The text asks us to consider which level of control would be the slowest in response time, and which would be the fastest. We are also asked which level of control would be most efficient in terms of the use of resources, and which would be the least efficient.
In class discussion, we guessed that the fastest response time would be achieved through post-translational control. Several examples from human biology came to mind. Consider blood clotting, which is controlled by proteolytic activation of a clot-forming protein that is abundant. For that matter, the sending of signals through nerves (very fast) is in part controlled by the interaction of proteins and small molecules with ion channel proteins. We agreed that the next-fastest response time would be through translational control. While a bacterial example does not immediately spring to mind, the eggs of many animals are loaded with mRNAs that are not translated until fertilization, allowing the rapid synthesis of proteins needed for development. We agreed that transcriptional control would have the slowest response time, requiring both the transcription of a gene and the translation of the mRNA.
The question of which method of regulation uses resources most efficiently turns these responses around. The most resource-efficient method of control is transcriptional control, where we don't even make mRNAs until they are needed, let alone synthesize proteins. Post-translational control would be the least efficient in terms of resources, as we have synthesized both mRNA and protein before producing the target for regulation. As in the real world, getting a fast response is expensive, and being very efficient about resource utilization means that the response will be slower.
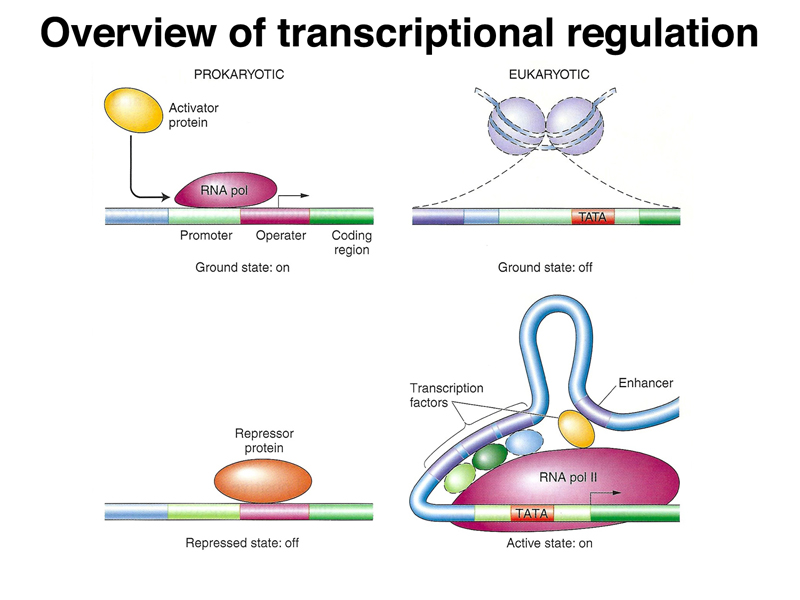
Before we get into the details of prokaryotic gene regulation, it is useful to compare gene regulation in prokaryotes and eukaryotes, as shown in the figure below. In prokaryotes, the chromosome is essentially naked DNA. The default or ground state is to allow transcription. Regulation of many prokaryotic genes is negative, meaning that a repressor protein binds at the 5' end of a gene to block transcription. In eukaryotes, chromatin is packed into nucleosomes, a complex of chromosomal DNA and basic proteins called histones. The default or ground state of eukaryotic genes is off, and a collection of transcription factors is necessary to activate a gene.
The figure below compares positive and negative regulation. In positive regulation, a transcriptional activator protein binds a region at the 5' end of a gene, called an operator, to promote transcription. In the absence of the activator protein, there is no transcription. In negative regulation, a repressor binds the operator sequence to prevent transcription. Only in the absence of repressor protein can the gene be transcribed.

One of the first examples of gene regulation to be worked out genetically was the lac operon of E. coli. This work was done by Francois Jacob and Jacques Monod in 1961. They received the Nobel Prize in 1965 in recognition of their work.
E. coli and other bacteria are very efficient at using available food sources. One of the cheapest (metabolically) food sources for E. coli is glucose, a monosaccharide that is at the core of central energy metabolism. The structure of glucose is shown below.

E. coli is capable of utilizing other sugars, such as lactose, a disaccharide present in milk. Lactose, as shown below, is a disaccharide of galactose and glucose. The first step in the utilization of lactose is the cleavage of lactose to galactose and glucose. The enzyme that carries out this reaction is β-galactosidase.

E. coli needs to make two proteins to utilize lactose, as shown below. In addition to β-galactosidase, it must also make a permease that allows lactose to pass through the plasma membrane.

We can ask how E. coli responds to the presence of lactose by plating bacteria on three different kinds of plates, as shown below: plates with glucose only, plates with glucose + lactose, and plates with lactose only.

The results are shown below. Cells plated on glucose do not make β-galactosidase. This makes sense, because in the absence of lactose this enzyme serves no purpose, and it is a waste of metabolic energy to synthesize the enzyme. Cells plated on glucose + lactose do not make β-galactosidase. This is a little surprising, but also makes sense when we consider that it is "cheaper" to grow on glucose than on lactose, so bacteria will not metabolize lactose in the presence of abundant glucose. Finally, cells plated on lactose alone make a lot of β-galactosidase in order to take advantage of lactose as an energy source.

The first step in the genetic analysis of the regulation of β-galactosidase is the isolation of mutant strains of bacteria that are unable to utilize lactose. This is readily accomplished using replica plating, as shown below. Mutagenized bacteria are grown on complete medium, which contains glucose, the replica-plated onto lactose and complete plates. As shown in the figure, we can identify colonies that can grow on glucose but not lactose by comparing the complete and lactose plates. Colonies present on the complete plate but missing from the lactose plate are unable to use lactose as an energy source. There are several different kinds of mutations that cause this phenotype. The easiest to understand are mutations in the β-galactosidase gene that eliminate the function of the enzyme. There are other kinds of mutations that eliminate the ability to grow on lactose, but we will defer discussion of these until we have looked at some other useful techniques.

We have seen that it is possible to induce β-galactosidase by growing cells in the presence of lactose and the absence of glucose. It is quite useful to have a compound that induces β-galactosidase that is not itself metabolized. Such a compound is isopropylthiogalactopyranoside or IPTG, shown below. IPTG is a gratuitous inducer of β-galactosidase. It has a molecular structure that looks like lactose to some kind of lactose-sensing element of E. coli cells, but can't be metabolized.

Another useful compound is nicknamed X-gal, shown below. X-gal looks something like lactose to the cell. It can be cleaved by β-galactosidase to release the chromogen (color-forming compound) on the right in the drawing. The chromogen forms an insoluble blue precipitate over colonies that are expressing β-galactosidase.

The image below shows a plate with colonies of E. coli treated with X-gal. Some colonies express β-galactosidase and are blue. Others do not express β-galactosidase and are white. This is a great trick. The plates typically contain IPTG as a gratuitous inducer as well.

Partial diploids of E. coli bearing F' plasmids that carry a portion of the E. coli genome that includes the genes for lactose utilization are also useful. We described the origin of F' plasmids in the previous lecture.
The genetic analysis of the regulation of β-galactosidase is fairly complex, so it is best to start with an overview of what is known before plunging into the details. The genes required for lactose utilization in E. coli are clustered into a single region on the chromosome. Besides β-galactosidase (lacZ) and permease (lacY), there is a third gene, transacetylase (lacA), that we will not describe further. The three genes are organized into an operon, as shown below. Organization into an operon means that the three genes are transcribed as a single mRNA that encodes all three proteins. There is another gene, lacI, that encodes a repressor protein. The repressor is a regulatory protein that binds to an upstream region of the lac operon called the operator. When the repressor is bound, the lac operon cannot be transcribed. Binding of an inducer (lactose or IPTG) to the repressor causes it to change shape so that it no longer binds the operator, and transcription of the operon can occur.

The figure below adds detail to the model. The lacI gene encodes the repressor, as shown. In the absence of inducer (lactose or IPTG) the repressor protein remains bound to the operator, preventing RNA polymerase from initiating transcription of the operon. In the presence of an inducer, the repressor no longer binds the operator and transcription occurs. The mRNA has three different start sites for the three different proteins. Ribosomes bind at three separate ribosome binding sites and initiate translation of three different proteins at separate start codons.
A student asked why the three genes are organized into an operon. In general, "why" questions are challenging in biology, but in this case we can say that organization into an operon allows the coordinate regulation of three genes involved in the same metabolic pathway. If the genes were separate, each would need its own regulatory signals. Organization of genes in the same metabolic pathway into operons is common in E. coli and other bacteria, but very uncommon in eukaryotes.
Another student asked if a nonsense or frameshift mutation in lacZ would affect the expression of lacY or lacA. Because each gene has its own ribosomal binding site and start codon on the mRNA, we can see that mutations in lacZ will not affect the expression of lacY or lacA. Even frameshift mutations in lacZ will not affect the expression of lacY or lacA, because the start codons for the downstream genes do not need to be in the same reading frame as lacZ.

Now that we have the overview of the lac operon, we are ready to look at some of the genetic evidence that led to the formulation of the model. The initial evidence was entirely genetic, although these days we have plenty of DNA sequence analysis and X-ray crystallography of proteins that support the model, some of which we wlll present.
The table below shows the synthesis of β-galactosidase (lacZ) and permease (lacY) in the presence and absence of the inducer IPTG. The data presented in the table are focused on a specific mutation in the operator called an operator-constitutive (lacOC).
| β-galactosidase (lacZ) | permease (lacY) | ||||
| Genotype | - IPTG | + IPTG | - IPTG | + IPTG | Conclusions |
| O+ Z+ Y+ | - | + | - | + | Wild type is inducible. |
| O+ Z+ Y+/F' O+ Z- Y+ | - | + | - | + | Z+ is dominant to Z-. |
| OC Z+ Y+ | + | + | + | + | OC is constitutive. |
| O+ Z- Y+/F' OC Z+ Y- | + | + | - | + | Operator is cis-acting. |
In the table above, the first line shows the behavior of wild type (O+ Z+ Y+). As we have previously described, synthesis of β-galactosidase (lacZ) and permease (lacY) proteins occurs only in the presence of the inducer. When the inducer is not present, these genes are not transcribed.
The second line shows the phenotype of a partial diploid bearing an F' plasmid with the lac genes. These cells are heterozygous for a mutation that eliminates the function of β-galactosidase (lacZ-). We can see that Z+ is dominant to Z-, exactly what we expect for a mutation in an enzyme. We have previously seen that mutations that eliminate the function of human enzymes are typically recessive (albinism, alkaptonuria, phenylketonuria, and biotinidase deficiency).
The third line shows the phenotype of an operator-constitutive mutation (lacOC). These cells are constitutive, that is, they synthesize β-galactosidase (lacZ) and permease (lacY) proteins in the absence of inducer.
The fourth line shows the phenotype of a partial diploid bearing an F' plasmid with the lac genes. In this case, the cells are heterozygous for both lacZ- and lacY-. The normal lacZ+ allele is on the F' plasmid next to an operator-constitutive allele (lacOC), while the normal lacY+ allele on the bacterial chromosome next to a normal operator. Notice that lacY is inducible while lacZ is constitutive. This demonstrates that the operator is cis-acting; the genes located on the same DNA molecule as the lacOC mutation are constitutive, while genes located on the same DNA molecule as the wild-type operator (lacO+) are inducible.
A model of the action of lacOC in partial diploids is shown below.

We now know the sequence of the operator, and the changes that occur in lacOC alleles. The operator is a rather short sequence that is palindromic. The single base changes in the operator sequence shown result in lacOC alleles.

The table below shows the synthesis of β-galactosidase (lacZ) and permease (lacY) in the presence and absence of the inducer IPTG. The data presented in the table are focused on a loss-of-function mutation in the repressor (lacI-).
| β-galactosidase (lacZ) | permease (lacY) | ||||
| Genotype | - IPTG | + IPTG | - IPTG | + IPTG | Conclusions |
| I+ Z+ Y+ | - | + | - | + | Wild type is inducible. |
| I- Z+ Y+ | + | + | + | + | lacI- is constitutive. |
| I+ Z+ Y+/F' I- Z+ Y+ | - | + | - | + | lacI+ is dominant to lacI-. |
| I- Z- Y+/F' I+ Z+ Y- | - | + | - | + | lacI+ is trans-acting. |
In the table above, the first line shows the behavior of wild type (I+ Z+ Y+). Synthesis of β-galactosidase (lacZ) and permease (lacY) proteins occurs only in the presence of the inducer. When the inducer is not present, these genes are not transcribed.
The second line shows the phenotype of lacI-. In the absence of the repressor, the cells are constitutive, that is, they synthesize β-galactosidase (lacZ) and permease (lacY) proteins in the absence of inducer.
The third line shows the phenotype of a partial diploid bearing an F' plasmid with the lac genes. These cells are heterozygous for lacI-, but are inducible. This shows that loss-of-function mutations in the repressor are recessive.
The fourth line shows the phenotype of a partial diploid bearing an F' plasmid with the lac genes. In this case, the cells are heterozygous for both lacZ- and lacY-. The normal lacZ+ allele is on the F' plasmid next to a normal allele (lacI+), while the normal lacY+ allele on the bacterial chromosome next to the mutant allele (lacI-). Both enzymes are inducible, which shows that the repressor, unlike the operator, is trans-acting.
A model of the action of lacI- in partial diploids is shown below.

The table below shows the synthesis of β-galactosidase (lacZ) and permease (lacY) in the presence and absence of the inducer IPTG. The data presented in the table are focused on a superrepressor mutation (lacIS).
| β-galactosidase (lacZ) | permease (lacY) | ||||
| Genotype | - IPTG | + IPTG | - IPTG | + IPTG | Conclusions |
| I+ Z+ Y+ | - | + | - | + | Wild type is inducible. |
| IS Z+ Y+ | - | - | - | - | lacIS is uninducible. |
| IS Z+ Y+/F' I+ Z+ Y+ | - | - | - | - | lacIS is dominant to lacI+. |
In the table above, the first line shows the behavior of wild type (I+ Z+ Y+). Synthesis of β-galactosidase (lacZ) and permease (lacY) proteins occurs only in the presence of the inducer. When the inducer is not present, these genes are not transcribed.
The second line shows the phenotype of lacIS. In the absence of the superrepressor, the cells are uninducible, that is, they cannot synthesize β-galactosidase (lacZ) or permease (lacY) proteins, even in the presence of inducer.
The third line shows the phenotype of a partial diploid bearing an F' plasmid with the lac genes. These cells are heterozygous for lacIS and are uninducible. This shows that the superrepressor mutation is dominant.
A model of the action of lacIS in partial diploids is shown below. The repressor encoded by the superrepressor allele does not respond to the presence of inducer, either because it cannot bind inducer or because the protein does not change shape to make it unable to bind to the operator. In the presence of a normal allele and inducer, all of the superrepressor protein will be bound to all of the available operators. The superrepressor is also trans-acting.

A student asked about the phenotype of cells bearing both an operator-constitutive mutation and a superrepressor allele. We know that the operator-constitutive mutation (lacOC) eliminates the ability of the repressor to bind to the operator. This would apply to the superrepressor allele (lacIS) as well, making this cell type constitutive. We can also think about what would happen in a partial diploid. The alleles in cis to the operator-constitutive mutation would be constitutive, while those in cis to the normal operator (lacO+) would be uninducible.
We leave as an exercise to the student to give the phenotype of the cells in the last three lines of the table below.
| β-galactosidase (lacZ) | permease (lacY) | ||||
| Genotype | - IPTG | + IPTG | - IPTG | + IPTG | Conclusions |
| I+ O+ Z+ Y+ | - | + | - | + | Wild type is inducible. |
| I+ OC Z+ Y+ | + | + | + | + | OC is constitutive. |
| IS O+ Z+ Y+ | - | - | - | - | lacIS is uninducible. |
| IS OC Z+ Y+ | ? | ? | ? | ? | ? |
| IS O+ Z- Y+/F' I+ OC Z+ Y- | ? | ? | ? | ? | ? |
| IS OC Z- Y+/F' I+ O+ Z+ Y- | ? | ? | ? | ? | ? |
The lac operon has a typical E. coli promoter with conserved sequences at -10 and -35. The figure below shows promoter mutations that have mild effects on transcription and those that have severe effects on transcription. Recall that the operator is downstream (toward the 3' end) of the promoter, so if the repressor is bound, RNA polymerase cannot initiate transcription.

At the beginning of the lecture, we saw that while the lac operon is induced in the presence of lactose alone, induction does not occur in the presence of both lactose and glucose. This is an example of the more generalized phenomenon of glucose repression, where the presence of glucose represses the expression of genes for the utilization of other, more "expensive" energy sources.
Glucose is not a corepressor in the sense that lactose is an inducer. Glucose does not bind to a regulatory protein to negatively regulate other operons. Rather, glucose inhibits adenylate cyclase, which synthesizes cyclic AMP from ATP. In the presence of high glucose, the cellular concentration of cAMP is low, while in the absence of glucose, the cellular concentration of cAMP is high, as shown in the figure below.

There is a protein called CAP (catabolite activator protein) in E. coli that binds cAMP. When CAP is bound to cAMP, it changes shape and becomes a positive regulator of the lac operon and other operons, as shown in the figure below.

As shown below, the lac operator site that we have discussed is distinct from the CAP binding site in the lac operon. Both are short palindromic sequences.

The image below shows a cartoon of the CAP-cAMP complex binding to DNA, as well as a model based on X-ray crystallography. You can see that CAP is in contact with both the -10 and -35 binding sites for RNA polymerase. Binding of CAP-cAMP to the promoter region facilitates the binding of RNA polymerase and greatly increases lac operon transcription when repressor is not bound.

The figure below shows how glucose repression works. The concentration of cAMP, a co-activator, is inversely correlated to the concentration of glucose. Again, "glucose repression" is something of a misleading term if we are focused on the actual mechanism of control of transcription. It might be better called "cAMP induction."

We briefly reviewed the genetic evidence for this model:
We have now seen two examples of the interaction of regulatory proteins, small molecules, and the control regions of the lac operon. One example, the lac repressor, is an example of negative regulation that is relieved by the presence of a small effector molecule (lactose or IPTG). The other example, CAP protein, is an example of positive regulation that is activated by the presence of a different small effector molecule (cAMP). The figure below presents these ideas in a more generalized form.

We are now ready to describe the state of the lac operon under the three plating conditions that we used to introduce the subject. The figure below shows cells in the presence of high glucose but no lactose. The level of cAMP is low, so CAP is not activated (absence of positive regulation). There is no lactose, so the lac repressor remains bound (negative regulation). Under these conditions there is no transcription of the lac operon.

The figure below shows cells in the presence of both glucose and lactose. The level of cAMP is low, so CAP is not activated (absence of positive regulation). In the presence of lactose, the lac repressor is no longer bound (absence of negative regulation). Under these conditions there is a low level of transcription of the lac operon.

The figure below shows the cells in the presence of lactose alone. The level of cAMP is high, so CAP is activated (positive regulation). In the presence of lactose, the lac repressor is no longer bound (absence of negative regulation). Under these conditions there is a very high level of transcription of the lac operon.

Here is the equivalent figure from your text showing the three conditions in the same order.
